Understanding the activation and inactivation of proteins is crucial in various fields, including biology, chemistry, and medicine. Proteins, the workhorses of our cells, undergo intricate processes to carry out their designated functions. This blog post will delve into the fascinating world of protein activation and inactivation, exploring the mechanisms, significance, and applications of these processes.
The Role of Proteins

Proteins are essential macromolecules that play a multitude of roles in living organisms. They are involved in virtually every cellular process, from structural support to enzymatic reactions and signal transduction. The unique sequence of amino acids in each protein determines its structure and, consequently, its function.
Proteins can adopt diverse shapes, ranging from simple linear chains to complex three-dimensional structures. This structural diversity allows proteins to interact with other molecules, catalyze reactions, and transmit signals within cells and between cells.
Protein Activation: Unlocking Functionality

Protein activation is a fundamental process that transforms an inactive protein into its active form, enabling it to perform its intended function. This activation process is often triggered by specific stimuli or environmental conditions, ensuring that proteins are activated only when needed.
Mechanisms of Protein Activation

There are several mechanisms by which proteins can be activated. Here are some common ones:
- Conformational Change: Many proteins exist in an inactive state due to their folded structure. Upon activation, they undergo a conformational change, exposing active sites or binding regions that were previously hidden.
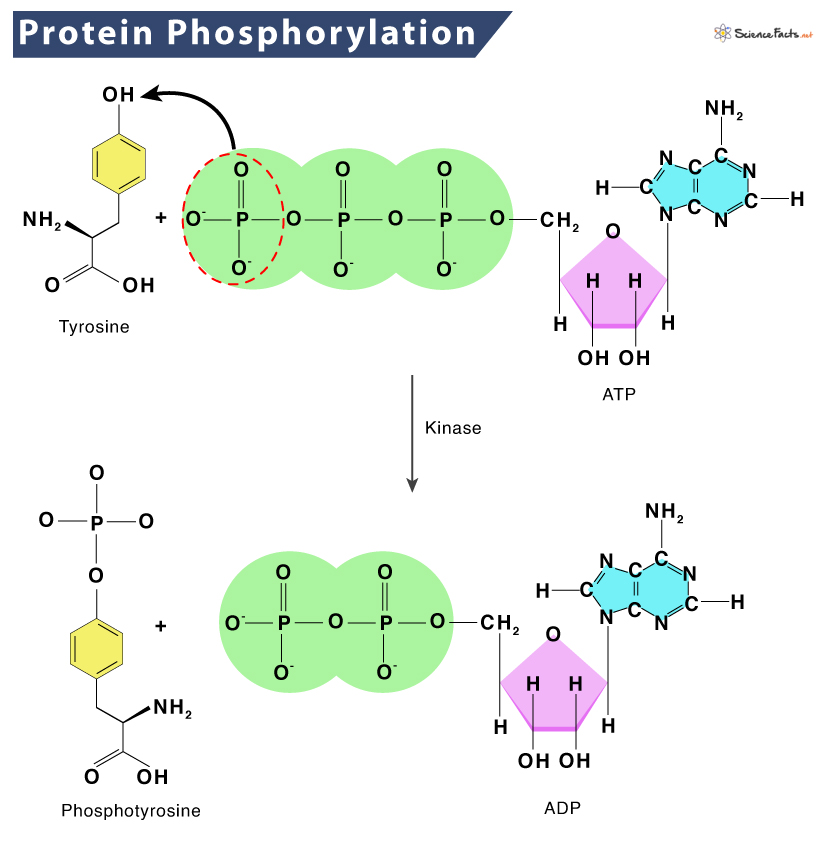
- Post-Translational Modifications: These modifications, such as phosphorylation, methylation, or acetylation, can alter the protein's structure and function. They often serve as regulatory mechanisms, fine-tuning protein activity.
- Binding to Co-factors or Substrates: Some proteins require specific co-factors or substrates to become active. For example, an enzyme may bind to its substrate, inducing a conformational change that activates its catalytic activity.
- Protein-Protein Interactions: Interactions between proteins can lead to activation. For instance, a signaling protein may activate another protein by binding to it, initiating a cascade of events.
Examples of Protein Activation

Let's explore a few well-known examples of protein activation:
- Enzymes: Enzymes are proteins that catalyze biochemical reactions. Many enzymes are activated by binding to their specific substrates, which induces a conformational change, exposing the active site.
- Receptor Proteins: Receptor proteins on cell surfaces can be activated by binding to specific ligands, such as hormones or neurotransmitters. This activation triggers a signaling cascade, leading to cellular responses.
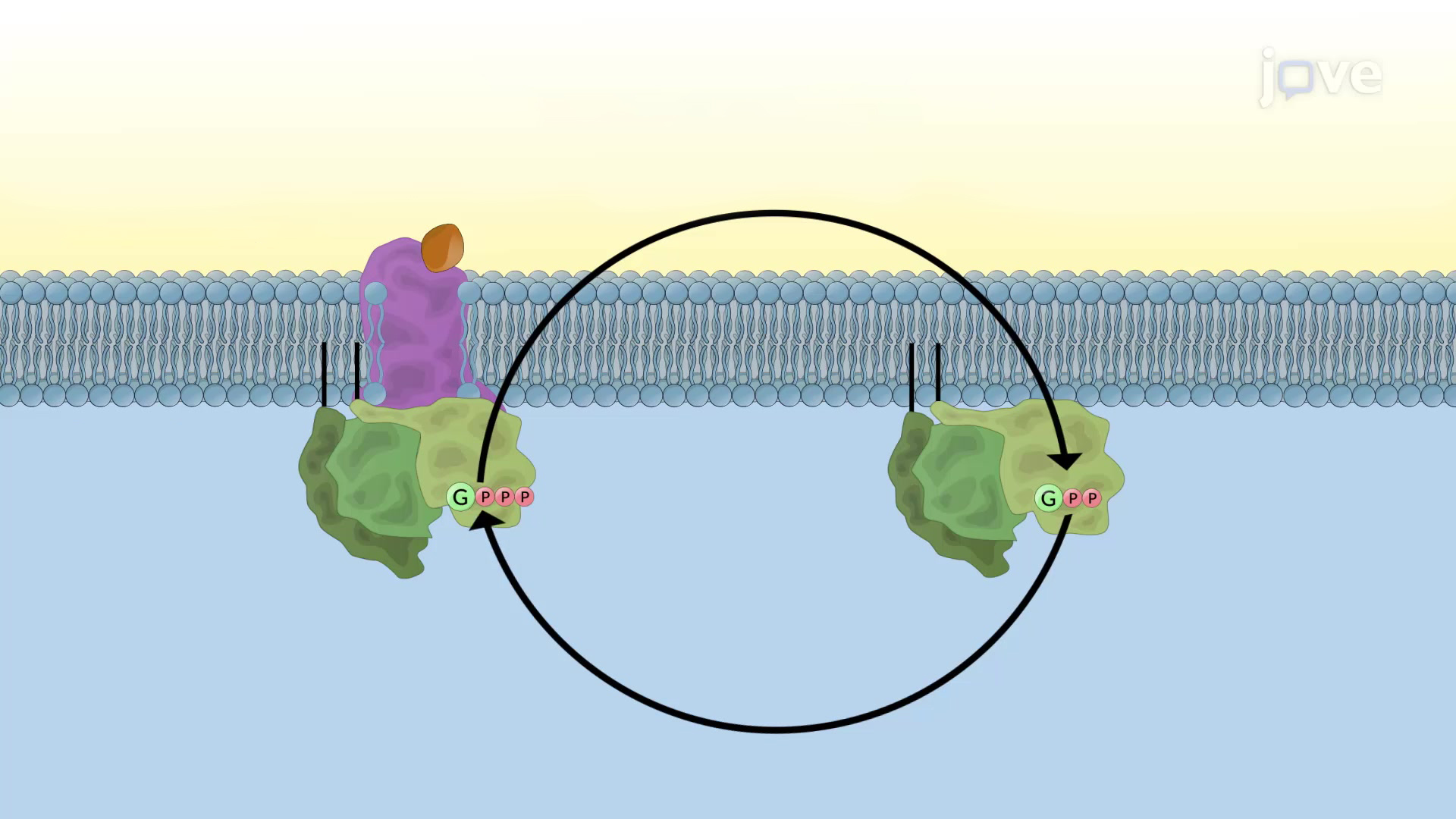
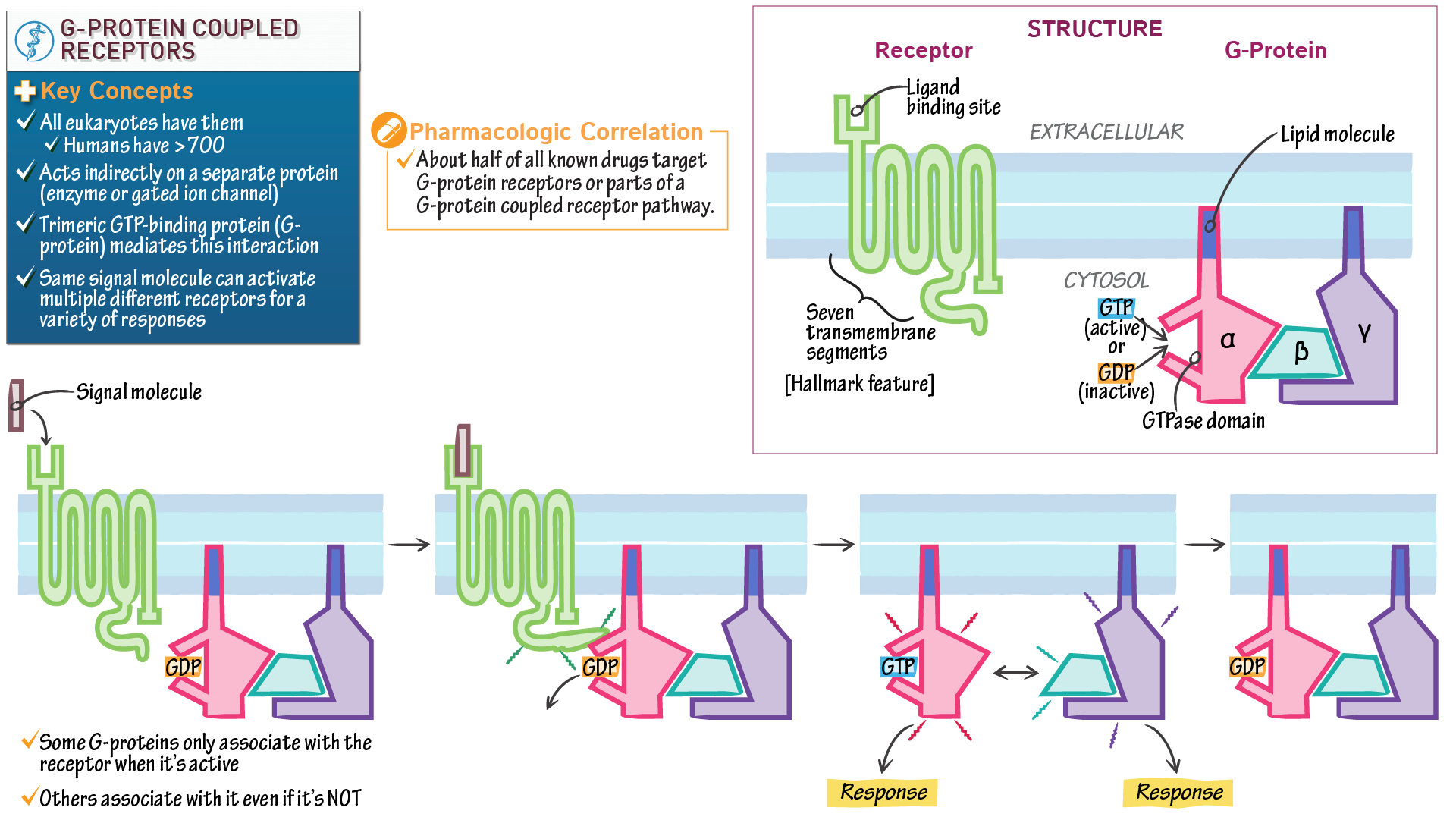
- G-Protein Coupled Receptors (GPCRs): GPCRs are a large family of receptors that play a crucial role in signal transduction. Upon binding to their ligands, GPCRs undergo conformational changes, activating intracellular signaling pathways.
Protein Inactivation: Regulating Activity

Protein inactivation is the process by which active proteins are rendered inactive, halting their function. This regulation is essential to prevent uncontrolled activity and maintain cellular homeostasis.
Mechanisms of Protein Inactivation

Protein inactivation can occur through various mechanisms, including:
- Conformational Change: Similar to activation, proteins can undergo conformational changes that mask active sites or binding regions, rendering them inactive.
- Degradation: Proteins can be targeted for degradation by cellular machinery, such as the proteasome. This process ensures the removal of damaged or unnecessary proteins.
- Deactivation by Regulatory Proteins: Some proteins are inactivated by interacting with regulatory proteins. For instance, phosphatases can reverse the phosphorylation of proteins, thereby inactivating them.
- Substrate Depletion: In certain cases, protein activity is regulated by the availability of substrates. When the substrate is depleted, the protein becomes inactive.
Examples of Protein Inactivation

Here are a few notable examples of protein inactivation:
- Enzyme Inhibition: Enzymes can be inhibited by competitive or non-competitive inhibitors, which bind to the enzyme and prevent it from interacting with its substrate.
- Protein Phosphatases: Protein phosphatases remove phosphate groups from phosphorylated proteins, reversing the activation process and rendering the protein inactive.
- Protein Folding Chaperones: Chaperone proteins assist in the proper folding of newly synthesized proteins. If a protein fails to fold correctly, it may be targeted for degradation, effectively inactivating it.
The Importance of Protein Activation and Inactivation

The activation and inactivation of proteins are vital for several reasons:
- Regulation of Cellular Processes: By controlling protein activity, cells can precisely regulate essential processes such as metabolism, cell growth, and differentiation.
- Homeostasis Maintenance: Protein activation and inactivation help maintain a stable internal environment, ensuring that cellular functions remain balanced.
- Response to Environmental Changes: Proteins can be activated or inactivated in response to external stimuli, allowing cells to adapt to changing conditions.
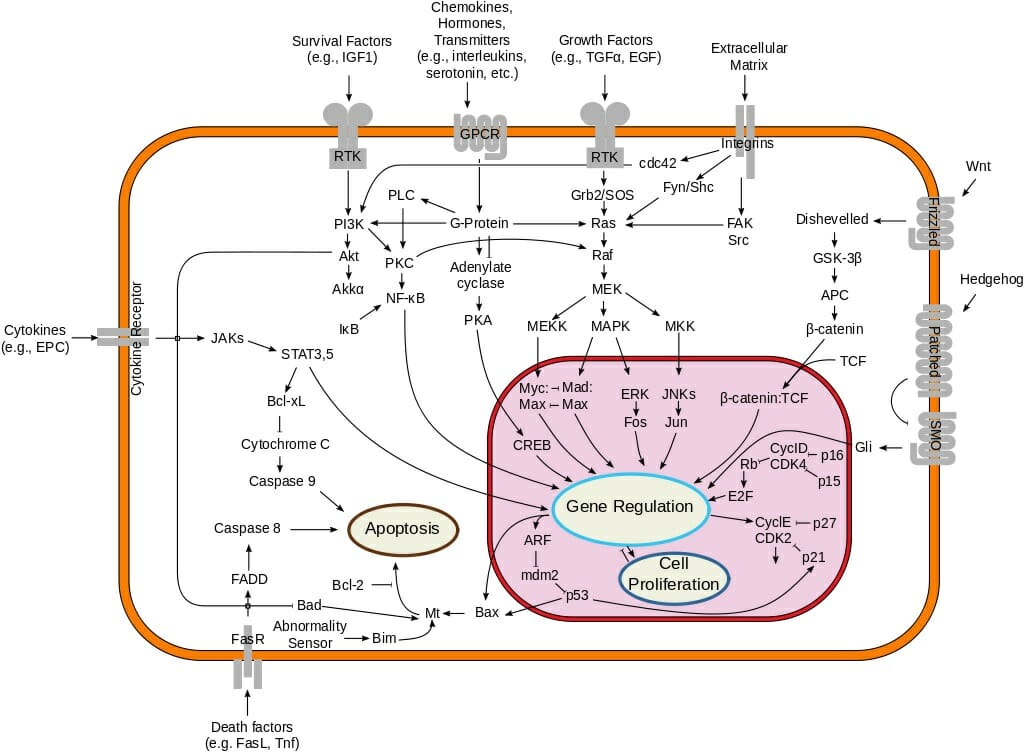
- Signal Transduction: The activation and inactivation of signaling proteins are crucial for transmitting information within cells and coordinating cellular responses.
Applications in Medicine and Biotechnology
A deep understanding of protein activation and inactivation has led to significant advancements in various fields:
- Drug Development: Many drugs target specific proteins to treat diseases. By modulating protein activity, researchers can develop effective therapies for conditions such as cancer, diabetes, and neurological disorders.
- Biotechnology: The activation and inactivation of proteins are exploited in biotechnology for various purposes, including enzyme engineering, protein purification, and the development of biofuels.
- Disease Diagnosis: The activity of certain proteins can serve as biomarkers for diagnosing diseases. For instance, elevated levels of specific enzymes may indicate liver or kidney dysfunction.
Conclusion

Protein activation and inactivation are intricate processes that underpin the functionality of living organisms. By unraveling the mechanisms behind these processes, scientists have gained valuable insights into cellular biology and developed innovative applications in medicine and biotechnology. The continuous study of protein dynamics promises to unlock even more exciting discoveries in the future.
Frequently Asked Questions
How do proteins activate and inactivate themselves without external stimuli?

+
Proteins can activate and inactivate themselves through internal regulatory mechanisms. For example, some proteins have auto-inhibitory domains that block their active sites until a specific stimulus triggers a conformational change.
Can a protein be both activated and inactivated simultaneously?
+
Yes, it is possible for a protein to exist in a state where certain regions are activated while others are inactivated. This dynamic regulation allows for precise control over protein function.
Are there any proteins that remain constantly active or inactive?
+
While most proteins undergo activation and inactivation, some proteins, such as structural proteins or certain enzymes, may remain in a relatively constant state of activity. Their function is more dependent on their presence rather than activation.
How do researchers study protein activation and inactivation in the lab?
+
Researchers employ various techniques to study protein activation and inactivation. These include biochemical assays, fluorescence-based methods, mass spectrometry, and genetic engineering approaches to manipulate protein activity.
Can protein activation and inactivation be reversed?
+
In many cases, protein activation and inactivation are reversible processes. For instance, an enzyme can be activated by binding to its substrate and inactivated by the removal of the substrate or the addition of an inhibitor. However, some protein modifications, such as certain types of cleavage, may be irreversible.