AlCl3, or aluminium chloride, is an interesting chemical compound with unique magnetic properties. Understanding its magnetic behavior is crucial for various scientific and industrial applications. In this blog post, we will delve into the world of AlCl3 and explore whether it is diamagnetic or paramagnetic.
The Magnetic Nature of AlCl3
Aluminium chloride, AlCl3, is a fascinating compound that exhibits intriguing magnetic characteristics. Its magnetic behavior is determined by the presence of unpaired electrons in its atomic or molecular orbitals. Let's unravel the magnetic nature of AlCl3 and discover its true identity.
Understanding Diamagnetism and Paramagnetism

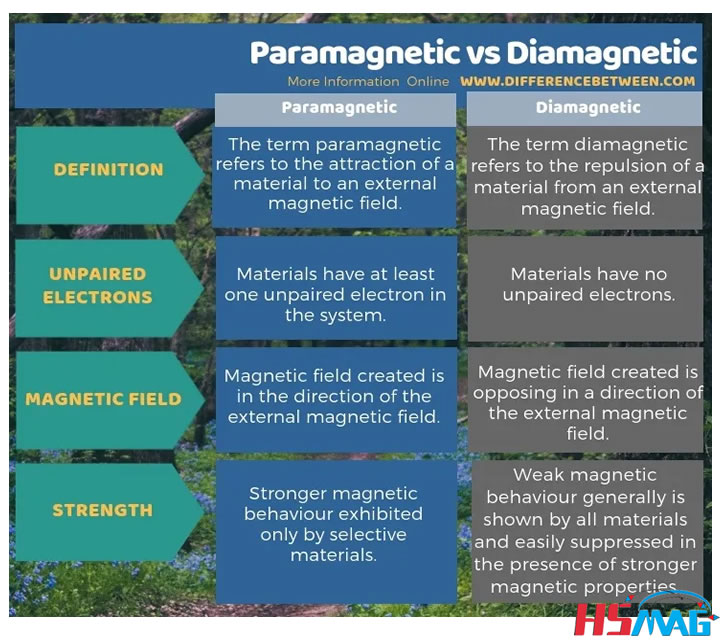
Before we dive into AlCl3, let's briefly discuss the concepts of diamagnetism and paramagnetism. These terms describe how substances respond to an external magnetic field.
Diamagnetic materials, such as copper and silver, have no unpaired electrons in their atomic orbitals. When exposed to an external magnetic field, they induce a weak magnetic field in the opposite direction, causing a repulsive force. This behavior is known as diamagnetism.
On the other hand, paramagnetic materials, like oxygen and aluminum, possess unpaired electrons. In the presence of an external magnetic field, these unpaired electrons align themselves with the field, resulting in an attractive force. This phenomenon is called paramagnetism.
AlCl3 - Diamagnetic or Paramagnetic?
Now, let's focus on AlCl3 and determine its magnetic behavior. Aluminium chloride is a covalent compound formed by the reaction of aluminium and chlorine. Its molecular structure consists of aluminium atoms bonded to chlorine atoms through covalent bonds.
To determine whether AlCl3 is diamagnetic or paramagnetic, we need to examine its electron configuration. Aluminium, with an atomic number of 13, has an electron configuration of [Ne]3s23p1. Chlorine, with an atomic number of 17, has an electron configuration of [Ar]3d104s24p5.
When aluminium and chlorine form AlCl3, the valence electrons of aluminium (3s23p1) and chlorine (4s24p5) combine to create a stable molecular structure. In this case, the three chlorine atoms form a trigonal planar arrangement around the central aluminium atom.
In AlCl3, all the valence electrons are paired up in the molecular orbitals. This means that there are no unpaired electrons available to align with an external magnetic field. As a result, AlCl3 exhibits diamagnetic behavior.
Diamagnetic substances, like AlCl3, are weakly repelled by magnetic fields. This repulsion occurs due to the induced magnetic field opposing the external magnetic field. It is a subtle effect and requires sensitive equipment to detect.
Experimental Verification

To confirm the diamagnetic nature of AlCl3, scientists have conducted various experiments. One common method is to use a magnetic susceptibility measurement technique. By placing a sample of AlCl3 in a magnetic field and measuring its response, researchers can determine its magnetic behavior.
Magnetic susceptibility measurements have consistently shown that AlCl3 exhibits negative susceptibility, indicating its diamagnetic nature. This negative susceptibility value confirms that AlCl3 is repelled by magnetic fields, just like other diamagnetic materials.
Applications of AlCl3

Understanding the magnetic properties of AlCl3 has practical implications in various fields. Here are some key applications:
- Catalysis: AlCl3 is widely used as a catalyst in the chemical industry. Its diamagnetic nature plays a crucial role in facilitating certain chemical reactions.
- Synthesis: Aluminium chloride is involved in the synthesis of various organic compounds. Its magnetic behavior influences the outcome of these reactions.
- Analytical Chemistry: AlCl3 is utilized in analytical techniques, such as chromatography, to separate and identify compounds based on their magnetic properties.
- Material Science: The magnetic characteristics of AlCl3 are studied to develop new materials with enhanced magnetic properties for various applications.
Safety Considerations

While AlCl3 is an interesting compound with unique magnetic properties, it is important to handle it with caution. Aluminium chloride is a highly reactive and corrosive substance. It can cause skin and eye irritation and should be handled in a well-ventilated area with appropriate personal protective equipment.
Always follow safety guidelines and consult the material safety data sheet (MSDS) when working with AlCl3 or any other chemical compound.
Conclusion
In conclusion, AlCl3, or aluminium chloride, is a diamagnetic compound. Its molecular structure and electron configuration result in the absence of unpaired electrons, leading to its weak repulsion by magnetic fields. Understanding the magnetic nature of AlCl3 is crucial for various scientific and industrial applications, from catalysis to material science.
By exploring the magnetic properties of substances like AlCl3, we gain a deeper understanding of the fundamental principles of chemistry and physics. This knowledge enables us to harness the power of magnetism for innovative technologies and advancements in multiple fields.
Is AlCl3 paramagnetic or diamagnetic in nature?

+
AlCl3 is diamagnetic due to the absence of unpaired electrons in its molecular structure.
How can I determine the magnetic behavior of a compound like AlCl3?
+
You can determine the magnetic behavior by examining the electron configuration and molecular structure of the compound. If there are no unpaired electrons, it is likely diamagnetic.
What are some practical applications of AlCl3 in the industry?
+
AlCl3 is used as a catalyst in various chemical reactions, in the synthesis of organic compounds, and in analytical chemistry techniques.
Are there any safety concerns when working with AlCl3?

+
Yes, AlCl3 is a highly reactive and corrosive substance. It can cause skin and eye irritation, so proper safety precautions and personal protective equipment are necessary.