The field of clinical research is an exciting and dynamic one, offering a range of career opportunities for those passionate about advancing medical knowledge and improving patient care. Among the key roles in this field, the Clinical Research Coordinator (CRC) stands out as a crucial position, ensuring the smooth and ethical conduct of clinical trials. In this blog post, we will delve into the world of Clinical Research Coordinator jobs, exploring their responsibilities, qualifications, and the impact they have on the healthcare industry.
Understanding the Role of a Clinical Research Coordinator

A Clinical Research Coordinator is a vital member of the research team, responsible for the day-to-day management and coordination of clinical trials. They work closely with investigators, research staff, and participants to ensure the trial's success and adherence to ethical guidelines.
Key Responsibilities of a CRC

- Trial Initiation: CRCs are involved from the early stages of a trial, assisting with protocol development, budget creation, and site selection.
- Participant Recruitment and Screening: Ensuring that eligible participants are identified and recruited, while maintaining strict confidentiality.
- Data Collection and Management: CRCs collect and manage data, ensuring its accuracy and integrity throughout the trial.
- Regulatory Compliance: They navigate the complex regulatory landscape, ensuring the trial meets all ethical and legal requirements.
- Study Progress Monitoring: CRCs track the progress of the trial, identifying potential issues and taking corrective actions as needed.
- Communication and Collaboration: Effective communication with investigators, research staff, and participants is crucial for a CRC's success.
The role of a Clinical Research Coordinator is diverse and challenging, requiring a unique set of skills and qualifications.
Qualifications and Skills for a Successful CRC Career

To excel as a Clinical Research Coordinator, certain qualifications and skills are essential:
Education and Training

- A bachelor's degree in a relevant field, such as biology, nursing, or healthcare administration, is often required.
- Some positions may prefer candidates with advanced degrees or certifications in clinical research.
- Continuing education and training in clinical research regulations and ethics are highly recommended.
Key Skills

- Attention to Detail: CRCs must be meticulous, ensuring accurate data collection and compliance with protocols.
- Organizational Skills: Managing multiple trials and tasks requires excellent organizational abilities.
- Communication: Effective communication is vital for building relationships with research teams and participants.
- Problem-Solving: CRCs often encounter challenges and must find creative solutions.
- Computer Proficiency: Proficiency in using clinical research software and databases is essential.
- Time Management: Juggling various responsibilities demands efficient time management skills.
These qualifications and skills form the foundation for a successful career as a Clinical Research Coordinator.
The Impact of Clinical Research Coordinators

The work of Clinical Research Coordinators has a profound impact on the healthcare industry and patient care. By ensuring the smooth conduct of clinical trials, CRCs contribute to the development of new treatments, therapies, and medical devices.
Advancing Medical Knowledge

Clinical trials are the backbone of medical research, and CRCs play a crucial role in their execution. Through their efforts, new insights into diseases and potential treatments are discovered, leading to advancements in medical knowledge.
Improving Patient Care

The work of CRCs directly benefits patients. By participating in clinical trials, patients gain access to cutting-edge treatments and contribute to the development of more effective therapies. CRCs ensure that participants receive the highest standard of care and that their rights and well-being are protected.
Ethical Conduct and Patient Safety

Clinical Research Coordinators are the guardians of ethical conduct and patient safety in clinical trials. They ensure that trials are conducted in accordance with strict ethical guidelines, protecting the rights and welfare of participants. CRCs also play a vital role in identifying and managing potential risks and adverse events, ensuring the safety of trial participants.
Career Opportunities and Growth

The field of clinical research offers excellent career opportunities and growth prospects for Clinical Research Coordinators. With experience and further education, CRCs can advance to senior coordinator roles, research manager positions, or even become principal investigators leading their own clinical trials.
Specialization and Expertise

CRCs can specialize in specific therapeutic areas, such as oncology, cardiovascular diseases, or rare disorders. This specialization allows them to become experts in their field, contributing to the development of targeted treatments and therapies.
Networking and Collaboration

The clinical research community is highly collaborative, providing CRCs with opportunities to network and collaborate with researchers, clinicians, and industry professionals. These connections can lead to new career paths and exciting research opportunities.
Challenges and Rewards of the CRC Role

While the role of a Clinical Research Coordinator is challenging, it also offers numerous rewards and a sense of fulfillment.
Challenges

- Regulatory Complexity: Navigating the complex regulatory landscape can be a significant challenge.
- Time Management: Balancing multiple trials and tasks requires effective time management skills.
- Participant Recruitment: Finding and enrolling eligible participants can be a difficult task.
- Adverse Events: Managing adverse events and ensuring participant safety is a critical responsibility.
Rewards

- Impact on Patient Care: Knowing that your work directly improves patient outcomes is immensely rewarding.
- Contribution to Medical Knowledge: Being part of groundbreaking research and advancing medical science is inspiring.
- Collaborative Environment: Working with a diverse team of researchers and clinicians fosters a sense of community.
- Professional Growth: The opportunity for continuous learning and career advancement is motivating.
Conclusion
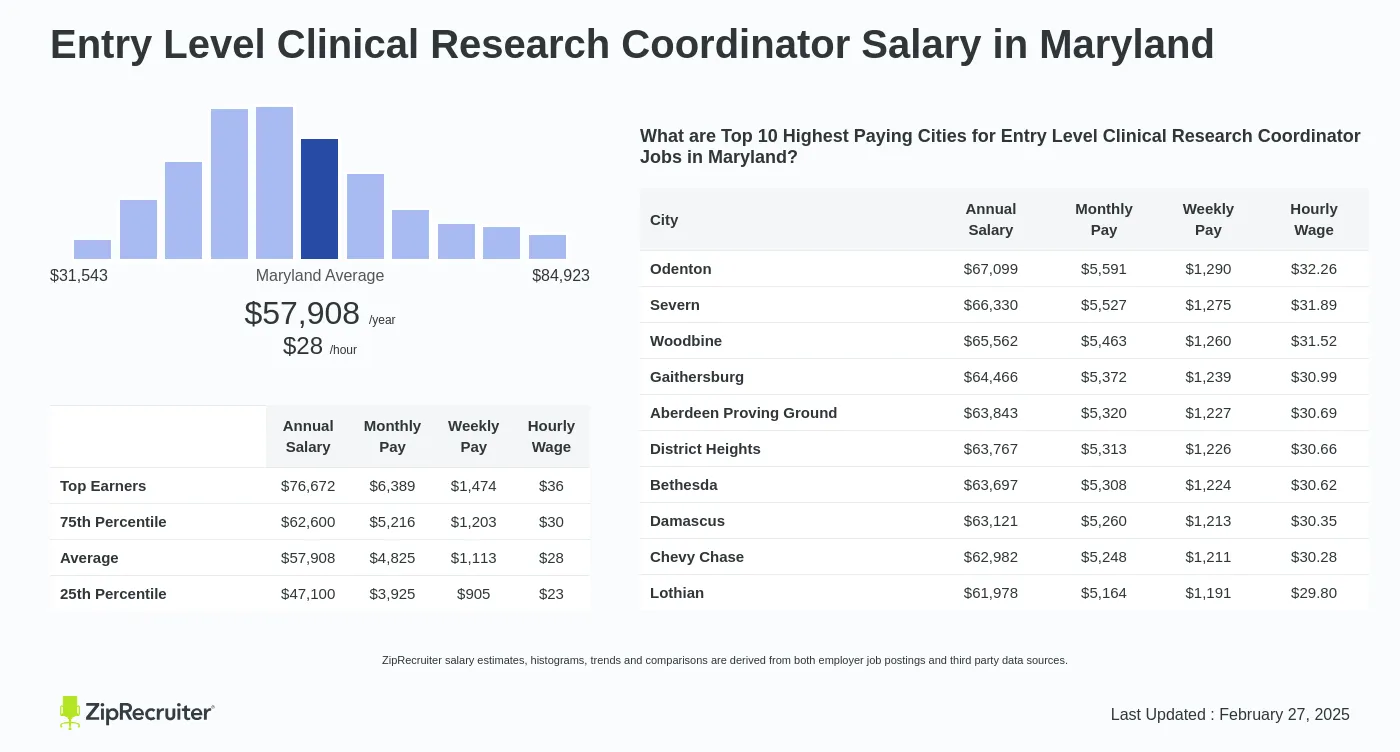
Clinical Research Coordinator jobs are integral to the success of clinical trials and the advancement of medical knowledge. The role demands a unique combination of skills, qualifications, and a dedication to ethical conduct. By managing and coordinating clinical trials, CRCs contribute to the development of life-changing treatments and therapies, ultimately improving patient care. If you are passionate about making a difference in healthcare and have an interest in research, a career as a Clinical Research Coordinator could be an exciting and rewarding path to explore.
What is the typical educational background for a Clinical Research Coordinator?
+A bachelor’s degree in a relevant field is typically required, with some positions preferring advanced degrees or certifications in clinical research.
How do Clinical Research Coordinators ensure ethical conduct in clinical trials?
+CRCs adhere to strict ethical guidelines, protect participant rights, and ensure informed consent. They also monitor trials for any ethical concerns or adverse events.
What are the career advancement opportunities for Clinical Research Coordinators?
+With experience and further education, CRCs can advance to senior roles, research management positions, or even become principal investigators.
How do Clinical Research Coordinators contribute to patient care?
+CRCs ensure that participants receive the highest standard of care, have access to cutting-edge treatments, and contribute to the development of new therapies.