The world of chemistry is vast and ever-expanding, with an array of chemical entities playing crucial roles in various industries and scientific research. Among these, the trialkyl sulfonium ion stands out as a unique and intriguing player, offering a range of properties and applications that are essential to modern science and technology.
In this blog post, we delve into the mysteries of the trialkyl sulfonium ion, exploring its structure, formation, and the diverse ways it impacts our world. By unraveling its secrets, we aim to provide a comprehensive understanding of this essential chemical entity and its significance in different fields.
Understanding the Trialkyl Sulfonium Ion

The trialkyl sulfonium ion, often denoted as R3S+, is a positively charged species that belongs to the class of sulfonium ions. It consists of a sulfur atom bonded to three alkyl groups (R), which are typically carbon-based chains or rings. This unique structure gives the ion its distinctive properties and makes it a versatile tool in chemistry.
Formation and Synthesis

The formation of trialkyl sulfonium ions can occur through various chemical reactions. One common method involves the reaction of alkyl halides with sulfur-containing compounds, such as thiols or sulfonium salts. This process, known as sulfonium ion synthesis, allows for the creation of a wide range of trialkyl sulfonium ions with different alkyl groups.
For example, the reaction between an alkyl halide (R-X) and a thiol (R'-SH) can result in the formation of a trialkyl sulfonium ion (R3S+) and a halide ion (X−). The specific reaction conditions and reagents used can influence the yield and selectivity of the desired trialkyl sulfonium ion.
Additionally, trialkyl sulfonium ions can be synthesized through the oxidation of thioethers or through the use of specialized catalysts, such as transition metal complexes, which facilitate the formation of these ions under specific reaction conditions.
Properties and Characteristics

Trialkyl sulfonium ions possess several key properties that make them valuable in various applications:
- Stability: These ions are relatively stable, especially when compared to other sulfur-containing species. Their stability arises from the strong sulfur-carbon bonds and the delocalization of the positive charge over the three alkyl groups.
- Solubility: Trialkyl sulfonium ions often exhibit good solubility in organic solvents, making them suitable for various chemical reactions and processes.
- Reactivity: While stable, trialkyl sulfonium ions can undergo a range of chemical reactions, including nucleophilic substitution and oxidation-reduction processes. This reactivity allows for their use in synthesis and as intermediates in complex chemical transformations.
Applications and Impact

The unique properties of trialkyl sulfonium ions have led to their widespread use in numerous fields, contributing to advancements in science and technology.
Organic Synthesis

Trialkyl sulfonium ions play a crucial role in organic synthesis, serving as versatile building blocks for the creation of complex molecules. Their ability to undergo specific chemical reactions makes them valuable intermediates in the synthesis of pharmaceuticals, agrochemicals, and fine chemicals.
For instance, trialkyl sulfonium ions can be used in the synthesis of natural products, such as alkaloids and terpenes, which have important biological activities. They also find applications in the development of novel materials, including polymers and organic semiconductors.
Biological and Medicinal Chemistry

In the realm of biological and medicinal chemistry, trialkyl sulfonium ions have shown promising potential. These ions can interact with biological targets, such as enzymes and receptors, due to their unique structure and reactivity.
Research has explored the use of trialkyl sulfonium ions as potential therapeutic agents, particularly in the development of anticancer and antimicrobial drugs. Their ability to target specific biological pathways and disrupt cellular processes makes them attractive candidates for further investigation.
Catalysis and Material Science

Trialkyl sulfonium ions have also found applications in catalysis and material science. They can act as catalysts in various chemical reactions, facilitating the formation of desired products with high selectivity and efficiency.
Additionally, these ions can be incorporated into materials, such as polymers and nanoparticles, to enhance their properties. For example, trialkyl sulfonium ions can improve the conductivity and stability of organic electronic devices, making them valuable components in the development of flexible displays and organic solar cells.
Environmental Chemistry

The environmental impact of trialkyl sulfonium ions is an area of growing interest. While these ions are generally considered non-toxic, their presence in the environment can have implications for ecological systems.
Research has focused on understanding the behavior and fate of trialkyl sulfonium ions in natural environments, such as soil and water bodies. This knowledge is crucial for assessing the potential environmental risks associated with their use and disposal.
Safety and Considerations

Despite their versatility and applications, it is essential to handle trialkyl sulfonium ions with caution. These ions can be reactive and, in some cases, may pose health and safety risks if not properly managed.
When working with trialkyl sulfonium ions, it is crucial to follow established safety protocols, including the use of appropriate personal protective equipment (PPE) and proper waste disposal practices. Researchers and industrial professionals should be well-versed in the specific hazards associated with the ions they are handling and take necessary precautions to ensure a safe working environment.
Conclusion
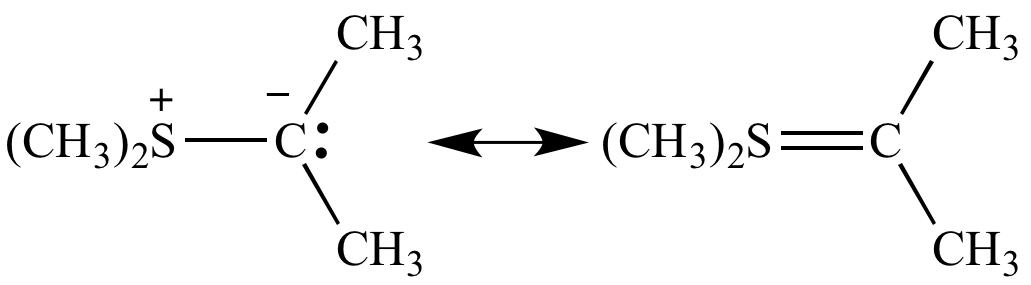
The trialkyl sulfonium ion is a fascinating chemical entity that continues to unlock new possibilities in science and technology. From its unique structure and formation to its diverse applications, this ion has become an essential tool in various fields, including organic synthesis, medicinal chemistry, catalysis, and material science.
As research progresses, we can expect further insights into the properties and behavior of trialkyl sulfonium ions, leading to innovative solutions and advancements. The exploration of this chemical entity highlights the endless potential of chemistry and its ability to shape our world.
What are the key characteristics of trialkyl sulfonium ions?

+
Trialkyl sulfonium ions are characterized by their stability, solubility in organic solvents, and reactivity in various chemical reactions. They are versatile building blocks in organic synthesis and have applications in biological and medicinal chemistry, catalysis, and material science.
How are trialkyl sulfonium ions synthesized?

+
Trialkyl sulfonium ions can be synthesized through the reaction of alkyl halides with sulfur-containing compounds, such as thiols or sulfonium salts. This process, known as sulfonium ion synthesis, allows for the creation of a wide range of trialkyl sulfonium ions with different alkyl groups.
What are the potential environmental impacts of trialkyl sulfonium ions?

+
While trialkyl sulfonium ions are generally considered non-toxic, their presence in the environment can have implications for ecological systems. Research focuses on understanding their behavior and fate in natural environments to assess potential environmental risks.
How are trialkyl sulfonium ions used in medicinal chemistry?

+
Trialkyl sulfonium ions have shown potential as therapeutic agents, particularly in the development of anticancer and antimicrobial drugs. Their ability to target specific biological pathways and disrupt cellular processes makes them attractive candidates for further investigation in medicinal chemistry.